Computational Medicine is an interdisciplinary research area with following A, B, C headings.
Science Writing & Communication. I am passionate about communicating our research and that of our peers to the broader scientific community as well as to the public. This is being done by means of blogs, magazines, as well as education journals.
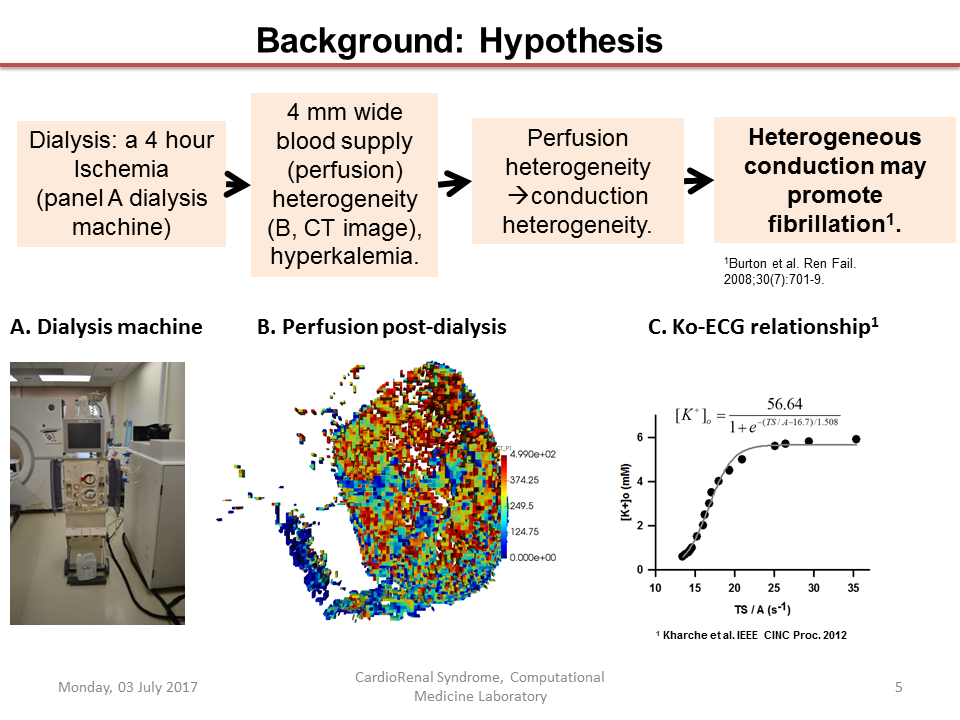
Dialysis prolongs life but augments cardiovascular mortality. Imaging data suggests that dialysis increases myocardial blood flow (BF) heterogeneity, but its causes remain poorly understood. A biophysical model of human coronary vasculature was used to explain the imaging observations, and highlight causes of coronary BF heterogeneity. Post-dialysis CT images from patients under control, pharmacological stress (adenosine), therapy (cooled dialysate), and adenosine and cooled dialysate conditions were obtained. The data presented disparate phenotypes. To dissect vascular mechanisms, a 3D human vasculature model based on known experimental coronary morphometry and a space filling algorithm was implemented. Steady state simulations were performed to investigate the effects of altered aortic pressure and blood vessel diameters on myocardial BF heterogeneity. Imaging showed that stress and therapy potentially increased mean and total BF, while reducing heterogeneity. BF histograms of one patient showed multi-modality. Using the model, it was found that total coronary BF increased as coronary perfusion pressure was increased. BF heterogeneity was differentially affected by large or small vessel blocking. BF heterogeneity was found to be inversely related to small blood vessel diameters. Simulation of large artery stenosis indicates that BF became heterogeneous (increase relative dispersion) and gave multi-modal histograms. The total transmural BF as well as transmural BF heterogeneity reduced due to large artery stenosis, generating large patches of very low BF regions downstream. Blocking of arteries at various orders showed that blocking larger arteries results in multi-modal BF histograms and large patches of low BF, whereas smaller artery blocking results in augmented relative dispersion and fractal dimension. Transmural heterogeneity was also affected. Finally, the effects of augmented aortic pressure in the presence of blood vessel blocking shows differential effects on BF heterogeneity as well as transmural BF. Improved aortic blood pressure may improve total BF. Stress and therapy may be effective if they dilate small vessels. A potential cause for the observed complex BF distributions (multi-modal BF histograms) may indicate existing large vessel stenosis. The intuitive BF heterogeneity methods used can be readily used in clinical studies. Further development of the model and methods will permit personalized assessment of patient BF status.
Link: Frontiers in Physiology, Computational 2018.
Abstract: The heterogeneity in the human left ventricle is augmented by heterogeneous perfusion defects in dialysis patients. We hypothesized that ischemic zones generated by heterogeneous perfusion are a cause of clinically observed post-dialysis arrhythmia. This preliminary study assessed the arrhythmia potential in a heterogeneously perfused 2D human ventricle computational model.
Our aim was to ascertain a relationship between the number of ischemia zones and incidence of multiple re-entrant waves in a 2D model of the human ventricle.
A human ventricle action potential model was modified to include the adenosine triphosphate (ATP) sensitive potassium current. Within ischemic zones, cell electrophysiological alterations due to ischemia were implemented as increased extracellular potassium, reduced intracellular ATP concentrations, as well as reduced upstroke current conductances. The cell model was incorporated into a spatial 2D model. The inter-cellular gap junction coupling was adjusted to simulate slow conduction in dialysis patient hearts. CT imaging data of the heart obtained during dialysis was analysed to estimate the approximate spatial size of ischemic zones. An ischemic border zone between the normal and central ischemic zones was implemented which had smoothly varying electrophysiological parameters. Arrhythmic potential was assessed using the paths of the centres of the re-entrant waves, called tip trajectories, and dominant frequency maps.
Extracellular potassium elevated the resting potential and IKATP reduced the action potential’s duration. In the absence of ischemic zones, the propensity of the model to induce multiple re-entrant waves was low. The inclusion of ischemic zones provided the substrate for initiation of re-entrant wave fibrillation. The dominant frequency which measured the highest rate of pacing in the tissue increased drastically with the inclusion of ischemic zones, going from 3 Hz in the pre-dialysis state to over 6 Hz in the post-dialysis state. Re-entrant wave tip numbers increased from 1 tip in the pre-dialysis case to 34 in the post-dialysis case, a 34 fold increase. The increase of tip number was found to be strongly correlated to tissue heterogeneity in terms of ischemic zone numbers. Computational factors limiting a more extensive simulation of cause-effect combinations were identified.
A dialysis session restores systemic homeostasis, but promotes deleterious arrhythmias. Structure-function mechanistic modelling will permit patient-specific assessment of health status. Such an effort is expected to lead to application of wider physical sciences methods in the improvement of the lives of critically ill patients. High performance computing is a crucial requirement for such mechanistic assessment of health status.
Authors: Sanjay R. Kharche, C W McIntyre.
Journal link: Lecture Notes in Computer Science, 2017 (HPCS 2017, Canada.)
Title: Computational assessment of the functional role of sinoatrial node exit pathways in the human heart.
Authors: Kharche SR, Vigmond E, Efimov IR, Dobrzynski HTitle: Effects of human atrial ionic remodelling by beta-blocker therapy on mechanisms of atrial fibrillation: a computer simulation.
Abstract
AIMS: Atrial anti-arrhythmic effects of beta-adrenoceptor antagonists (beta-blockers) may involve both a suppression of pro-arrhythmic effects of catecholamines, and an adaptational electrophysiological response to chronic beta-blocker use; so-called 'pharmacological remodelling'. In human atrium, such remodelling decreases the transient outward (Ito) and inward rectifier (IK1) K(+) currents, and increases the cellular action potential duration (APD) and effective refractory period (ERP). However, the consequences of these changes on mechanisms of genesis and maintenance of atrial fibrillation (AF) are unknown. Using mathematical modelling, we tested the hypothesis that the long-term adaptational decrease in human atrial Ito and IK1 caused by chronic beta-blocker therapy, i.e. independent of acute electrophysiological effects of beta-blockers, in an otherwise un-remodelled atrium, could suppress AF.
METHODS AND RESULTS: Contemporarily, biophysically detailed human atrial cell and tissue models were used to investigate effects of the beta-blocker-based pharmacological remodelling. Chronic beta-blockade remodelling prolonged atrial cell APD and ERP. The incidence of small amplitude APD alternans in the CRN model was reduced. At the 1D tissue level, beta-blocker remodelling decreased the maximum pacing rate at which APs could be conducted. At the three-dimensional organ level, beta-blocker remodelling reduced the life span of re-entry scroll waves.
CONCLUSION: This study improves our understanding of the electrophysiological mechanisms of AF suppression by chronic beta-blocker therapy. Atrial fibrillation suppression may involve a reduced propensity for maintenance of re-entrant excitation waves, as a consequence of increased APD and ERP.
Keywords: Arrhythmia; Atrial fibrillation; Computer simulation; Ion channel; beta-Blockers.
Link: Europace 2014 DOITitle:A mathematical model of action potentials of mouse sinoatrial node cells with molecular bases
Authors: Kharche S et al.
Abstract: Genetically modified mice are popular experimental models for studying the molecular bases and mechanisms of cardiac arrhythmia. A postgenome challenge is to classify the functional roles of genes in cardiac function. To unveil the functional role of various genetic isoforms of ion channels in generating cardiac pacemaking action potentials (APs), a mathematical model for spontaneous APs of mouse sinoatrial node (SAN) cells was developed. The model takes into account the biophysical properties of membrane ionic currents and intracellular mechanisms contributing to spontaneous mouse SAN APs. The model was validated by its ability to reproduce the physiological exceptionally short APs and high pacing rates of mouse SAN cells. The functional roles of individual membrane currents were evaluated by blocking their coding channels. The roles of intracellular Ca(2+)-handling mechanisms on cardiac pacemaking were also investigated in the model. The robustness of model pacemaking behavior was evaluated by means of one- and two-parameter analyses in wide parameter value ranges. This model provides a predictive tool for cellular level outcomes of electrophysiological experiments. It forms the basis for future model development and further studies into complex pacemaking mechanisms as more quantitative experimental data become available.
Keywords: computer modeling, cardiac action potential, genetic isoforms, pacemaking mechanisms.
Journal link/DOI: Am J Physiol Heart Circ Physiol. 2011 Sep; 301(3): H945–H963.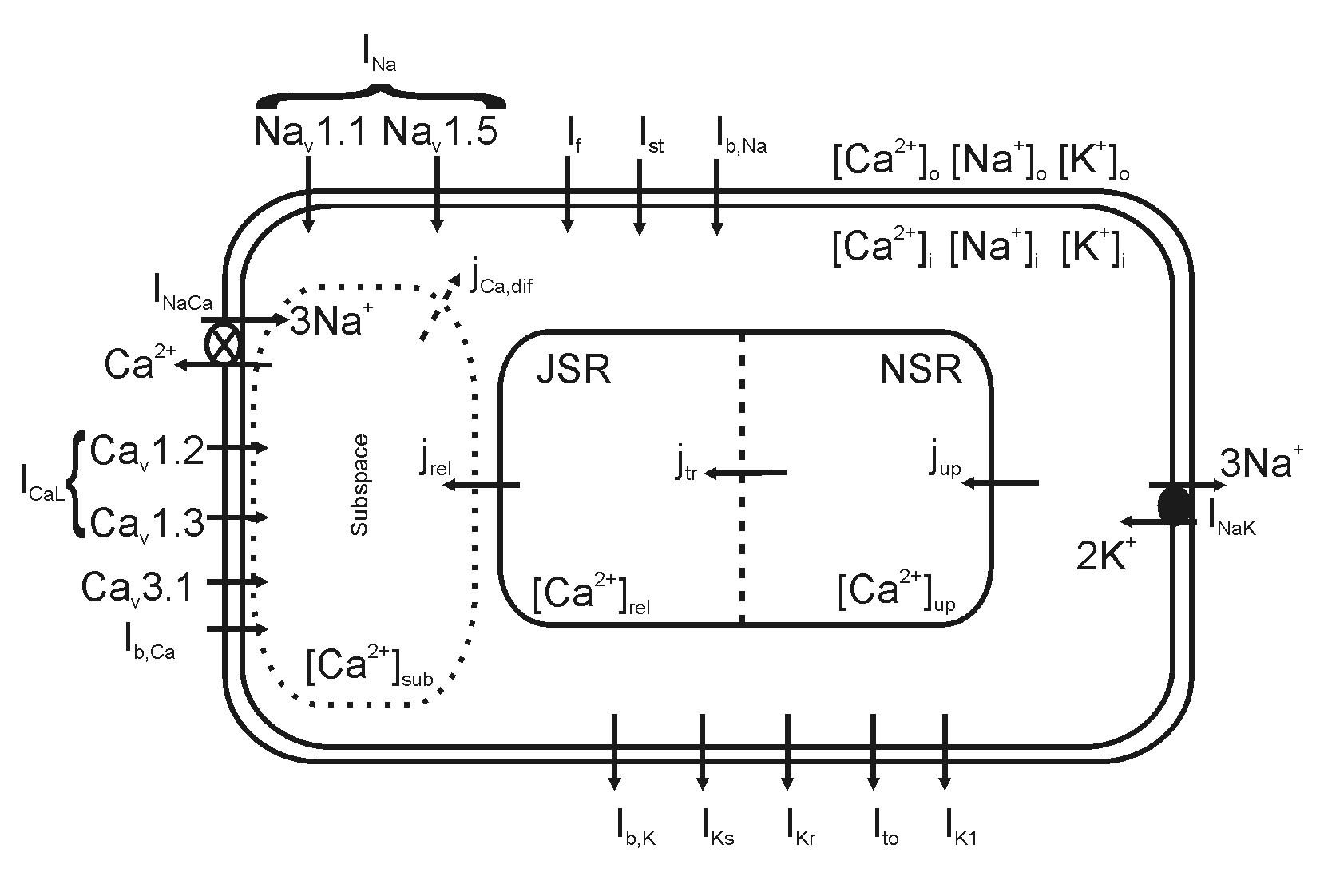
Title:Simulation of clinical electrophysiology in 3D human atria: a high-performance computing and high-performance visualization application.
Authors: Kharche, S; Seemann, G; et al.
Abstract: Atrial fibrillation (AF) is a common cardiac disease of genuine clinical concern with high rates of morbidity, leading to major personal and National Health Service costs. Computer modelling of AF using biophysically detailed cellular models with realistic 3D anatomical geometry allows investigation of the underlying ionic mechanisms in far more detail than with experimental physiology. We have developed a 3D virtual human atrium that combines detailed cellular electrophysiology including ion channel kinetics and homeostasis of ionic concentrations with anatomical details. The segmented anatomical structure and the multi-variable nature of the system make the 3D simulations of AF computationally large and intensive. Computational demands are such that a full problem-solving environment requires access to resources of high-performance computing (HPC), high-performance visualization (HPV), remote data repositories and backend infrastructure. This is a classic example of eScience and Grid-enabled computing. This study was carried out using multiple processor shared memory systems and massively parallel distributed memory systems. With the envisaged increase in anatomical and molecular detail in our cardiac models the requirement for HPC resources is predicted to increase many fold (similar to 1-10 teraflops). Distributed computing is essential, both through massively parallel systems (a single supercomputer) and multiple parallel systems made accessible through the Grid. Analysis and interpretation of results are enhanced by HPV, which in itself is a large data computing aspect of cardiac modelling.
Keywords:3D anatomically detailed model; Computer Science, Software Engineering; Computer Science, Theory &; Methods; biophysically detailed model; large-scale simulations
Journal link:DOI to WileyTitle: A Global Sensitivity Index for Biophysically Detailed Cardiac Cell Models: A Computational Approach
Authors: Sanjay R. Kharche, Niklas Ludtke, et al.
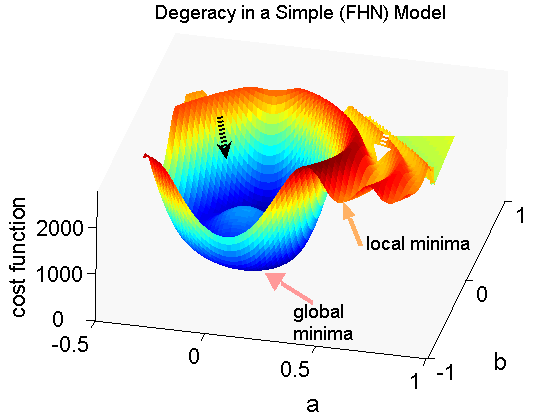
Abstract: Biophysically detailed cardiac cell models are based upon stiff ordinary differential equations describing ionic channels and intracellular dynamics aiming at reproducing experimental action potentials (APs) and intracellular calcium (Ca2+) transients. Channel blocking and bifurcation analyses are local sensitivity analyses in model parameter space. However, all parameters influence model behaviour and require a global sensitivity index quantifying the influence of parameters on model responses. Identification of the influence of individual parameters increases our understanding of models. A global parameter sensitivity index for assessing the sensitivity of model responses to parameters in cardiac cell models is proposed. The robust index was applied to four widely used models. The analysis revealed that whilst models have common sets of parameters influencing AP and transients, there are subtle differences. This sensitivity analysis offers a systematic method for quantifying the influence of individual parameters on model behaviour to assist in model reduction, refinement or development.
Keywords: Cardiac simulation, global sensitivity analysis, high throughput computing, modelling and simulation tool.
Journal link:Springer